Robinia pseudoacacia L.
 |
| Black Locust flowering in mid-June at Baker Park Reserve, Maple Plain, MN. |
Black Locust, also called False Acacia or Yellow Acacia, is
a medium to large tree originally from the Appalachian and Ozark mountains.
Intentionally planted for its strong wood, stabilizing roots and attractive flowers,
it has spread widely from its native range. It’s now found in all lower 48
states, several Canadian provinces and every continent around the globe (1, 2).
Black Locust History and Habitat
 |
Black Locust thrives in full sun. It reproduces quickly from root suckers. |
It’s unsettling, this march across the state. Black Locust
has escaped plantings to become naturalized, and where this adaptable plant finds
sun and anything but waterlogged soils, it survives and even thrives. Old
fields, rights of way and degraded woods are now part of its expanded habitat.
So are prairies, savannas and open forests, where single species stands
of Black Locust can challenge restoration (4, 5, 6).
Adapted for Colonization
Black Locust is aided in its spread by its pioneer habit, a
set of adaptations for quick colonization of canopy gaps and other
disturbances. It grows fast and produces abundant seeds carried by wind, water
and gravity. Although seeds may drop and germinate below their parent plants, within
a stand the trees reproduce mainly by vigorous root suckers and stump sprouts, shoots
that arise from roots and trunks. That’s especially true if the trees are
damaged, such as from a storm or from cutting, and explains why Black Locust is
so resilient and persistent.
What’s more, Black Locust is a legume, a member of the bean
family, Fabaceae. Like other plants in that family, it can fix nitrogen. Nodules
on its roots hold bacteria that can convert nitrogen gas in the atmosphere to
ammonia, a usable form. That’s an advantage in nutrient-poor soils, and not
just for Black Locust. Soils enriched with this captured nitrogen support other
plants, including non-native ones that may further displace native species (7).
The Neonative Debate
For these reasons, even inside its native range Black Locust is known to be weedy. Outside its range it’s often called invasive, but some biologists stop short of calling it non-native. Because its historical range is in North America, they prefer to call it a neonative, a species that isn’t native (here before Europeans) but also isn’t non-native (from another continent).
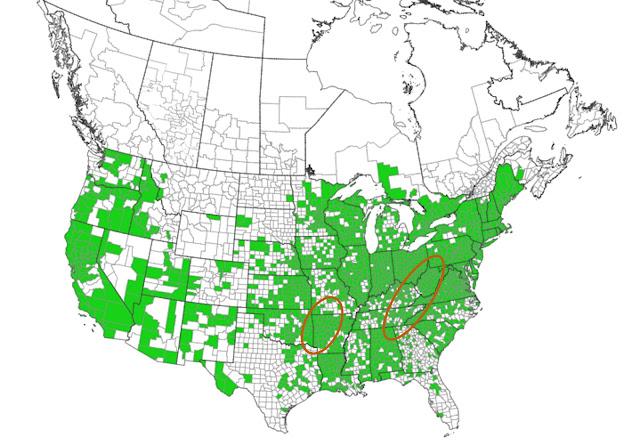 |
| Black Locust's distribution is in green, from EDDMapS (17). Approximate native range is added and circled in red, based on a map from the USDA Southern Research Station (18). |
As it was originally defined, a neonative species is one that moves to a new area in response to an environmental change, such as a warming climate (8). Such species arrive without direct human intervention, and because they come on their own, they challenge our ideas about which species belong in an area and which don’t – and therefore which should stay, and which shouldn’t (9).
Black Locust doesn’t quite fit that definition. Humans brought it here, so it’s not a neonative in the original sense of the word. The label stuck, though, and now Black Locust, the “nuisance neonative,” has a mixed reputation. In this area it’s bad for biodiversity, especially in natural areas, but in its home range it’s good for native insects and other animals (10). In the Midwest the nitrogen it adds to soils can alter natural communities in undesirable ways, but where mine lands need to be reclaimed, nitrogen enrichment aids recovery. And where forests and prairies need protection, Black Locust’s suckering growth is a problem, but where soils are eroding, the trees’ clutching roots are a solution (19).
Overseas, a Similar Story
The debate is just as vigorous in Europe. Black Locust was introduced there in the 1600s and now is found in more than 40 countries. It has been widely planted, and although it’s considered one of the worst invasive plants in the continent, it’s also valued for biomass production, erosion control and honey-making (11, 12). Biologists there recommend a “tiered approach” to managing Black Locust: Remove it from natural areas but keep it in commercial forests, in urban plantings and in selected forests where succession, the gradual change in plant community composition, is allowed to play out (12).
Winter Identification
Mature Black Locust hold 3- to 4-inch-long, brown pods through
winter. Each contains 4-8 seeds. Buds are alternate, but they’re under the leaf
scars and barely visible. Nodes often have a pair of stout thorns, ½ to 1 ½
inches long. Branches and young trunks may also have thorns. Mature bark is
dark gray or brown with deep furrows and flat-topped ridges.
 |
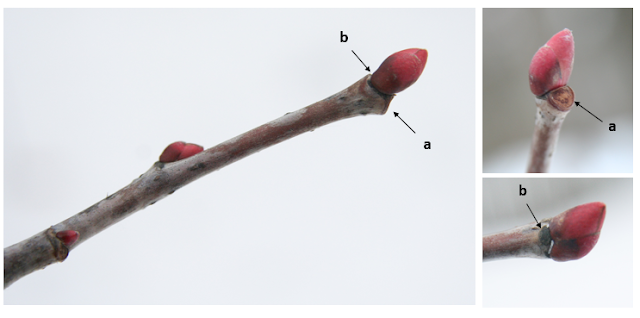
| Black Locust pods hang on mature trees through winter. Each pod is 3-4 inches long. |
 |
Black Locust buds are alternate but lie under the leaf scars, which may be cracked on their surfaces. A pair of stout thorns is found at most nodes. |
 |
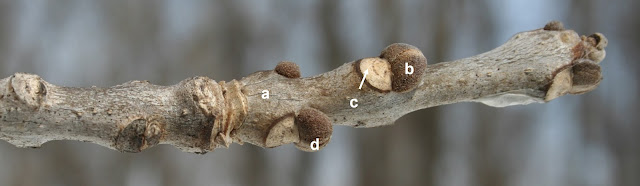
Mature bark is dark brown or gray with deep furrows and flat-topped ridges. Smaller trunks bear thorns. |
Look-alikes
Prickly Ash (Zanthoxylum americanum) is a shrub or small tree that also has pairs of thorns at the nodes, but the thorns are only ¼ to ½ inch long. Buds are red, fuzzy and clearly visible. Prickly Ash does not produce pods.
From a distance, Kentucky Coffee Tree (Gymnocladus
dioicus) resembles Black Locust. Pods on mature females are larger than
those of Black Locust and filled with a green mash. The tree has no thorns.
Honey Locust (Gleditsia triacanthos), likely
introduced to Minnesota as cultivated varieties, also has persistent pods, but they’re
much longer than Black Locust – about 16 inches. Some trees have large,
three-parted thorns on their trunks (13).
 |
Prickly Ash buds are red and fuzzy. The pair of thorns at the nodes are smaller than those of Black Locust |
 |
| Female Kentucky Coffee Trees carry pods through winter, but they're much larger than those of Black Locust. Seeds are embedded in a sticky, green mash. |
Regulation
The Minnesota Department of Agriculture includes Black
Locust on its list of Restricted noxious weeds, meaning the plant can’t be
imported, sold or transported except as allowed by state law (14). The rules are similar in Wisconsin (15).
Black Locust Toxicity
Leaves, bark and seeds contain robin and phasin, compounds
that interfere with protein synthesis and can kill cells. Horses are especially
sensitive to these toxins, but humans can also get sick with nausea, weakness,
flushing and lethargy. If any part of the plant is ingested, seek medical help
immediately (16).
References
1) USDA, NRCS. 2023. The PLANTS Database (http://plants.usda.gov, 02/02/2023). National Plant Data Team, Greensboro, NC USA.
2) Robinia pseudoacacia (black locust). CABI. CABI Compendium, https://doi.org/10.1079/cabicompendium.47698. Accessed Feb. 2, 2023.
3) University of Minnesota Bell Museum Minnesota Biodiversity Atlas. https://www.bellmuseum.umn.edu/atlas/ Accessed Feb. 2, 2023.
4) Minnesota Department of Natural Resources. Black locust (Robinia pseudoacacia). Accessed Feb. 2, 2023.
5) Minnesota Department of Agriculture. Black Locust. Accessed Feb. 2, 2023.
6) Woody Invasives of the Great Lakes Collaborative. Black locust. Accessed Feb. 2, 2023.
7) Von Holle, B., Neill, C., Largay, E.F., et al. 2013. Ecosystem legacy of the introduced N2-fixing tree Robinia pseudoacacia in a coastal forest. Oecologia 172: 915-924. https://doi.org/10.1007/s00442-012-2543-1
8) Essl, F. et al. 2019. A conceptual framework for range-expanding species that track human-induced environmental change. Bioscience 69 (11): 908-919. https://doi.org/10.1093/biosci/biz101
9) Shah, S. 2020. Native Species or Invasive? The Distinction Blurs as the World Warms. Yale E360.
10) Jaffe, D. Rethinking Black Locust. Posted in 2019 on the website for the Ecological Landscape Alliance.
11) Sitzia, T., Cierjacks, A., de Rigo, D., and Caudullo, G. 2016. Robinia pseudoacacia in Europe: distribution, habitat, usage and threats. In European Atlas of Forest Tree Species. Ed: San-Miguel-Avanz, J., de Rigo, D., Caudullo, G., Durrant, T., and Mauri, A. Publication Office of the European Union, Luxembourg.
12) VítkováM., Müllerová, J., Sádlo, J., Pergl, J., Pyšek, P. 2017. Black locust (Robinia pseudoacacia) beloved and despised: A story of an invasive tree in Central Europe. Forest Ecology and Management 384: 287-302. DOI: 10.1016/j.foreco.2016.10.057
13) Smith, W.R. 2008. Trees and Shrubs of Minnesota. University of Minnesota Press, Minneapolis.
14) Minnesota Department of Agriculture. Minnesota Noxious Weed List. Accessed Feb. 6, 2023.
15) Wisconsin Department of Natural Resources. Black Locust. Accessed Feb. 6, 2023.
16) Poison Control. Are Black Locust Trees Toxic? National Capital Poison Center. Accessed Feb. 9, 2023.
17) EDDMapS. 2023. Early Detection & Distribution Mapping System. The University of Georgia - Center for Invasive Species and Ecosystem Health. Available online at http://www.eddmaps.org/; last accessed February 11, 2023.
18) Huntley, J.C. No date. Black Locust. USDA Southern Research Station.
19) Farmer, S. 2020. Black Locust & Drought. CompassLive, USDA Southern Research Station.